Who should input to HTA decision-making?
Patient engagement
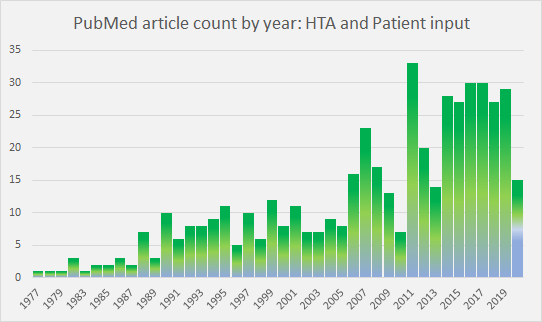
Patient input to HTA decision-making as a subject in the literature has peaked and plateaued in recent years (Figure 1). As a consequence of research and initiatives, such as those noted at the end of this article, the rationale for patient involvement in HTA is now well understood.
However, there is still a way to go in implementation.
Wale and Sullivan (2020) explore how patient input was reflected in the final documentation of HTA decisions by NICE, SMC and CADTH for a chronic disease and a cancer treatment considered during 2016-17. They discuss the influence of where in the process input is sought; how it is communicated (directly or written); by whom (individual patients or patient advocacy group); as well as the content of (clinical or realities of the disease beyond data); on the level of impact achieved. These and other opportunities to better involve patients in the HTA process were identified by Biointelect during research and interviews with NICE, SMC and CADTH, as well as PBAC stakeholders, for the BMS commissioned ‘Broadening the Evidence Report‘ (June 2019) (Figure 2).
In terms of the PBAC, Public Summary Documents (PSDs) currently include ‘Consumer comments’ in the Section titled ‘Consideration of the evidence’. The number and source of submissions received are noted, usually along with a summary of key points and a comment on usefulness to the Committee. No direct feedback is given to those who provide input. This may change with the most recent PBS Listing Procedure Guidance (V 1.7, April 2020) noting that the Consumer Input process is ‘under review’. The 2019 establishment of a designated ‘Consumer Evidence and Engagement Unit‘ in the Department of Health to ‘focus on expanding opportunities for consumers and patients to be central to ensuring that robust decision making can also support better transparency and understanding of HTA decision making processes‘, is likely involved in such changes. The recently launched Medicine Status Website was the product of considerable collaboration within and outside the Department.
Time for the public voice?
Street and colleagues (2020) say the terminology used to describe community participation in HTA, ‘is contested and frequently confusing‘. Depending on the jurisdiction, the terms: patients and consumers (with and without the advocate suffix), public, lay members, customers, clients, and citizens are used, often interchangeably. The article identifies the lack of a specific definition of the ‘public’ in the context of HTA, and propose the following:
‘A community member who holds the public interest and has no commercial, personal, or professional interest in the HTA process.’
This leads to the question, are ‘public’ and ‘patient’ engagement currently being viewed as the same thing during HTA processes? The patient perspective is lauded as it provides insights that clinical data often does not. That is invaluable to decision makers. However, where is the public voice, or more importantly, public values in the decision? Street (2020) notes that there is little empirical evidence on which to determine how different patient and public values are in this context. Patients may have a higher tolerance of risk, and, understandably an interest limited to a specific population and treatment. The public preference is towards equitable distribution amongst all patient groups, and the utilitarianism aim to maximise the well being of all.
In acknowledging this potential difference, UK’s NICE has established a Citizens Council. The Council is made up of 30 members of the public representative of UK demographics. It meets once a year and provides a perspective on relevant moral and ethical issues. The Council’s recommendations are incorporated into NICE principles and, where appropriate, into NICE’s methodology.
In the absence of the public perspective, do we assume that consideration of public values in HTA-based decision-making is the role of elected officials, in this case, the Minister of Health and Cabinet, who are the ultimate arbitrators of how to spend taxpayers funds? This will become increasingly important to understand as the pressure of unsustainable demands, driven by an ageing population and new technologies, require further rationing of healthcare costs.
References
- Wale JL, Sullivan M. Exploration of the visibility of patient input in final recommendation documentation for three health technology assessment bodies. International Journal of Technology Assessment in Health Care. 2020:1-7. https://doi.org/10.1017/S0266462320000240.
- Street J, Stafinski T, Lopes E, Menon D (2020). Defining the role of the public in Health Technology Assessment (HTA) and HTA-informed decision-making processes. International Journal of Technology Assessment in Health Care. 2020;36:87–95. https://doi.org/10.1017/S0266462320000094. Note: The Supplement provides a country-by-country summary of public and patient involvement in HTA and HTA-decision making and provides an excellent overview.
- Gagnon MP, Desmartis M, Lepage-Savary D, et al. Introducing patients’ and public’s perspectives to health technology assessment: A systematic review of international experiences. International Journal of Technology Assessment in Health Care. 2011;27:31-42.
- Hunter A, Facey K, Thomas V, et al. EUPATI Guidance for Patient Involvement in Medicines Research and Development: Health Technology Assessment. Front. Med. 2018;5:231. doi: 10.3389/fmed.2018.00231
Patient engagement initiatives
- Values and Standards for Patient Involvement in HTA (HTAi 2014);
- European Patients’ Academy on Therapeutic Innovation (EUPATI). A public-private partnership of industry, academia, not-for-profit, and patient organisations published guidance (Hunter 2016);
- the PREFER partnership guidelines for industry, Regulatory Authorities and HTA bodies on how and when to include patient perspectives on benefits and risks of medicinal products; and
- US National Health Council‘s focus on ensuring the voice of the patient and patient organizations are an integral part of the value discussion.
- the Patient Voice Initiative, established in 2015 (link to 2017 Report);
- Medicines Australia commenced a Patient Advocacy Working Group in 2019;
- Tip sheet for consumers to make comments to the PBAC.
