Will reimbursement authorities make Real World Evidence (RWE) mandatory?
Since 1990, members of the ICH [1] have co-operated to develop guidelines and standards for demonstration of the efficacy, safety and quality of new products. As membership has grown, the guidance documents have been increasingly adopted as the international standard. Consistently satisfying the data needs of reimbursement agencies is yet to reach this level of uniformity. Will authorities agree on, and even mandate, inclusion of RWE into reimbursement submissions?

Reimbursement guidelines ensure that all relevant evidence published in the medical and grey literature is identified and presented systematically for evaluation. In the case of a new medicine, this usually is comprised of the clinical trial data generated for regulatory purposes. HTA landscape reviews across a number of therapeutic areas demonstrate that positive reimbursement decisions continue to rely almost exclusively on direct randomised controlled trials (RCTs) [2]. Exceptions are rare disorders and conditions of high clinical need when decisions are pragmatically made on the available data. Compromised reimbursement decisions may also occur when regulatory approval is given on the basis of single arm studies; and ethics approval for an RCT requires cross-over to be permitted after the primary endpoint.
The landscape reviews also show that the inclusion of analyses based on real-world data (RWD) definitely aid in reducing uncertainty around a decision. The price decrease required from what is “cost-effective” for listing being inversely proportional to the size of the arc of uncertainty. Uncertainty is significantly reduced when an included analysis demonstrates clinical effectiveness [3]; estimates local usage parameters and/or supports applicability of RCT results to the proposed patient population. However, this importance only applies only once a “yes” funding decision has been made.
This is why development of the reimbursement strategy for a new product must start early. In concert with regulatory plans, it is critical to identify the limitations/gaps in the clinical development program relative to the competitive landscape; to determine which type of data will most strengthen the evidence base; and to seek out alternate sources of data to generate this evidence in a timely manner.
The context for inclusion of real-world evidence (RWE) includes initial reimbursement discussions, pharmacoeconomic analyses, and conditional reimbursement schemes [4]. The right RWE can optimise restrictions or conditions associated with a listing, as well as the price achieved. This occurs at both a global and local level from data collection during early access programs, commissioning a clinical audit of hospital records, to requesting ad hoc analyses of a disease registry.

A snapshot of the status of RWE in reimbursement authorities:
Asia
Dr Hwee-Lin Wee from the National University of Singapore, presented a session on Real-World Data/Evidence use by Asia’s developing HTA systems during the ISPOR Asia Pacific 2020 Conference. The results of a 2019 survey confirmed that HTA agencies accept clinical effectiveness data from real-world sources as supplementary evidence to RCTs or where RCTs are lacking. Specifically, China, Indonesia, Japan, Malaysia, Singapore, South Korea and Thailand accept pragmatic clinical trials. China, Philippines, Singapore and South Korea publish specific guidance documents on use of RWD/RWE in HTA reviews [5].
This work contributed to the REAL World Data In ASia for HEalth Technology Assessment in Reimbursement (REALISE) working group. This is a collaboration between global experts and 11 Asian health systems. The group has developed non-binding guidance to provide a framework to generate and use real-world data (RWD) / real-world evidence (RWE) in a consistent and efficient manner for drug reimbursement decision-making in Asia [6].
Latam
During 2016, the Karolinska Institute, CHE York, University Departments from Argentina, Brazil, Columbia and Chile, ICON and Novartis collaborated to systematically evaluate the sources, characteristics and uses of RWE in South America. Following comprehensive literature and database searches and validation workshops during 2016, Justo and colleagues (2019) found that RWE was being utilised for pharmacovigilance and academic research purposes; little for HTA decision making and pricing negotiations and not at all to inform early access schemes [7].
North America
USA
The FDA has published guidance on the use of RWE to support regulatory decision-making for medicines and medical devices. These data may also support coverage decisions and to develop guidelines and decision support tools for use in clinical practice [8, 9, 10].
Lee et al. (2021) [11] conducted a retrospective analysis of the use of RWE in the cost-effectiveness analysis and budget impact analysis sections of final evidence reports published in the database of the Institute for Clinical and Economic Review (ICER). They identified 33 reports, all of which used RWE, most commonly for disease progression inputs (28.7%) and health care resource utilization and costs (21.1%). In 57% of cases, a retrospective cohort study design was used to collect the data, registry data was the most frequent (41%) data source, and 30% of RWE was industry-sponsored. RWE was rarely used for drug-specific clinical outcomes such as effectiveness (1.5%), adverse drug event rates (0.5%), and discontinuation rates (1.2%).
Malone et al. (2018) [12] interviewed 20 healthcare professionals from 18 different US health organisations about the use of RWE to inform Pharmacy and Therapeutic Committee decisions. Overall, RWE was considered useful, in particular to inform safety monitoring, utilization management, and cost analysis, but less so to guide coverage decisions. When it was used in decision making, pharmacy claims data was referred to by 100% of committees represented; medical claims (80%), Electronic Health Records (27%); Consumer surveys (20%) and Patient registries (13%). The usefulness of published RWE depended on the relevance and applicability, transparency of methods, study design and quality, and timing of any results. Perceived organizational barriers to the use of published RWE included lack of skill, training, and timely study results.
The FDA has also produced a number of guidance documents on communication with payors around RWE [13, 14] and between manufacturers and payors such as formulary committees [15].
Canada
In response to the need for consistency and integration of RWE efforts, a 4-year collaboration between researchers, payers, and patients known as the Canadian Real-world Evidence for Value of Cancer Drugs (CanREValue [16], https://cc-arcc.ca/canrevalue ) kicked off in 2017. Five working groups are developing and testing a framework for Canadian provinces to harmonise the generation and use of RWE for cancer drug funding. The aim is to build consistency in the use of RWE at a national level, which will lead to a robust pan-Canadian system supporting sustainability, value for money and improved patient care.
As part of this project Clausen and colleagues (2020) [17] conducted a qualitative descriptive study to understand current issues with the use of RWE in cancer drug funding decisions. The findings suggest that if RWE is to be used in drug funding decisions, there is a need for a cultural shift, improved data infrastructure, committed investment in capacity building and increased stakeholder collaboration.
Europe
The value of using RWE in regulatory decision making by the European Medicines Agency (EMA) is well established [18]. The inclusion of RWE in benefit-assessment/HTA evaluations continues to be the subject of numerous initiatives.
The LSE conducted a series of roundtable meetings in 2018 [19] to gain an understanding of the use of RWE across Europe and to assist the pharmaceutical industry to enhance their use of RWE. Three ways to advance were identified:
(a) prioritising the use of RWE by focusing on quality, including credibility of sources, design and methodologies,
(b) by identifying key areas or regions to pilot the use of RWE, and identifying supporting stakeholders, and
(c) by securing a consistent approach by developing an action bias, building best practice case studies, and demonstrating the value of RWE in contributing to decision making.
In a review of RWE policies of the HTA agencies of Sweden, UK, Germany, France, Italy and The Netherlands, Malady et al (2017) [4] found a lack of alignment in request for and acceptance of RWD in contexts of initial reimbursement discussions, pharmacoeconomic analysis and conditional listing schemes. Bullement et al. (2020) [20] found that the use of RWE in NICE submissions of cancer drugs was extensive, and appeared to have provided a valuable source of information to aid the decision making. NICE continues to proactively develop its use and acceptance of RWE. In 2019, a ‘Widening the evidence base’ statement set out NICE’s ambition to use broader sources of data and analytic methods to enhance existing methods and processes. Last year, NICE announced a collaboration with US based Flatiron [21] to explore how RWE can inform the clinical and cost effectiveness of health technologies.
Internationally
The value of data collected outside of the clinical trial environment is clearly accepted by both payers and manufacturers with potential for use in evaluation and decision-making at all stages of the life cycle of a new medicine.
Representative bodies such as ISPOR and the International Society for Pharmacoepidemiology (ISPE) are collaborating to address the common issues noted as barriers to use of RWE. For example, recommending establishment of a register for RWE study protocols to improve transparency in research methods and analyses use. [22, 23] Numerous others continue to make valuable recommendations on how to move forward. [24, 25]
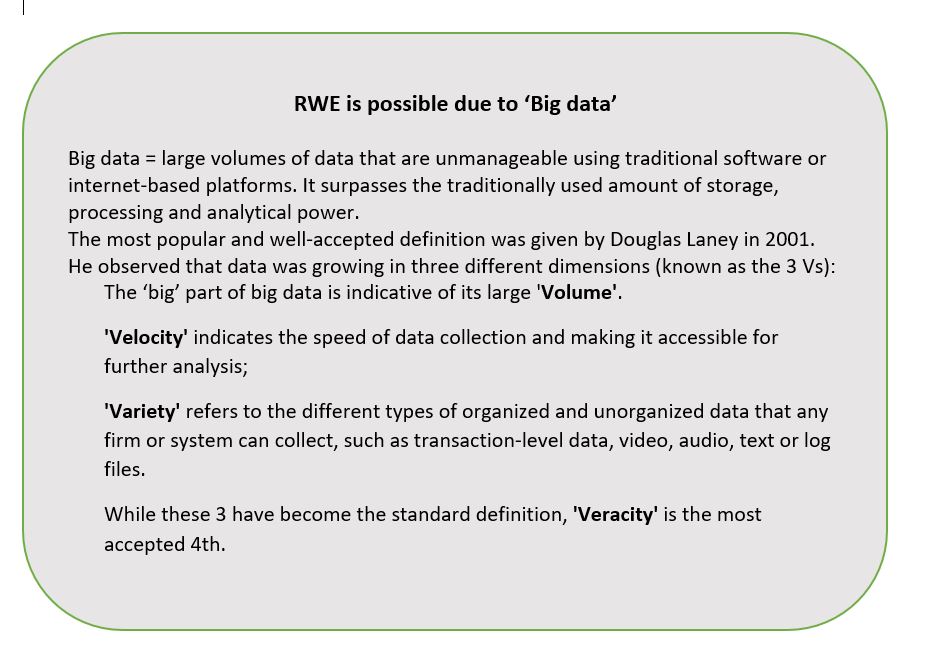
Policy consistency across organisations and jurisdictions is evitable. However, this will require moving beyond the established principles of evidence-based medicine into HTA 2.0, made possible by “big data” (see separate box). Additionally, new definitions of privacy and data security are required in an era where a search engine can know more about your health than your doctor.
Predictions are for a global market for sourcing, generating and providing RWD in a usable format of US$1.64 billion by 2024 [26]. As reimbursement authorities continue to receive more RWE of increasingly higher quality and value, it will become expected.
- The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use.
- Data on file
- The observed discrepancy between the effects of a health intervention in routine clinical practice (effectiveness) and the effects demonstrated in RCTs (efficacy) is known as the ‘efficacy effectiveness gap’. NICE Statement of Intent. Widening the evidence base: use of broader data and applied analytics in NICE’s work. https://www.nice.org.uk/Media/Default/About/what-we-do/NICE-guidance/NICE-guidelines/how-we-develop-nice-guidelines/statement-of-intent.docx Page 9.
- Makady A et al. Policies for Use of Real-World Data in Health Technology Assessment (HTA): A Comparative Study of Six HTA Agencies. Value Health. 2017 Apr;20(4):520-532. doi: 10.1016/j.jval.2016.12.003
- Lou et al. Real-world data for health technology assessment for reimbursement decisions in Asia: current landscape and a way forward. International Journal of Technology Assessment in Health Care 2020 https://doi.org/10.1017/S0266462320000628
- REAL World Data In ASia for HEalth Technology Assessment in Reimbursement (REALISE) working group. For Doers of HTA: HTA Agencies and HTA Researchers. Use of Real-World Data and Real-World Evidence to Support Drug Reimbursement Decision-Making in Asia. Full Version 01.11.2020. https://hiper.nus.edu.sg/wp-content/uploads/2020/12/REALISE-Full-guidance_updated-20201101.pdf
- Justo et al. (2019) Real-World Evidence in Healthcare Decision Making: Global Trends and Case Studies from Latin America. Value Health 2019;22(6):739-749.
- US FDA. Real-world data (RWD) and real-world evidence (RWE) are playing an increasing role in health care decisions. 30/11/2020. www.fda.gov/science-research/science-and-research-special-topics/real-world-evidence, accessed 11 Feb 2021.
- US FDA. Guidance Document. Submitting Documents Using Real-World Data and Real-World Evidence to FDA for Drugs and Biologics Guidance for Industry, May 2019. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/submitting-documents-using-real-world-data-and-real-world-evidence-fda-drugs-and-biologics-guidance
- US FDA. Use of Real-World Evidence to Support Regulatory Decision-Making for Medical Devices, issued 31/08/2017 https://www.fda.gov/files/medical%20devices/published/Use-of-Real-World-Evidence-to-Support-Regulatory-Decision-Making-for-Medical-Devices—Guidance-for-Industry-and-Food-and-Drug-Administration-Staff.pdf
- Lee Wj et al. Use of real-world evidence in economic assessments of pharmaceuticals in the United States. J Manag Care Spec Pharm. 2021;27(1):5-14. Thanks to Neil Grubert for sharing this article on the “Future of Pharmaceutical Market Access” LinkedIn group, see https://www.linkedin.com/posts/neil-grubert_use-of-rwe-in-economic-assessments-of-drugs-activity-6768935679868055552-pq87
- Malone et al. Real-World Evidence: Useful in the Real World of US Payer Decision Making? How? When? And What Studies? Value In Health 2018;21(3):326–333. https://doi.org/10.1016/j.jval.2017.08.3013
- Neumann PJ, Elle P. Cures Act, FDA draft guidance suggest flexibility on communication of real-world drug impacts, though questions remain. 2017. Accessed December 13, 2020. https:// www.healthaffairs.org/do/10.1377/ hblog20170202.058584/full/
- Neumann PJ, Weissman H. The FDA’s new guidance on payer communications: implications for real-world data and value-based contracts. 2018. Accessed December 13, 2020. https:// www.healthaffairs.org/do/10.1377/ hblog20180712.816686/full/
- U.S. Food and Drug Administration. Drug and device manufacturer communications with payors, formulary committees, and similar entities— questions and answers: guidance for industry and review staff. 2018. Accessed December 13, 2020. https:// www.fda.gov/regulatory-information/ search-fda-guidance-documents/ drug-and-device-manufacturer-communications-payors-formulary-committeesand-similar-entities
- Chan K et al. Developing a framework to incorporate real-world evidence in cancer drug funding decisions: the Canadian Real-world Evidence for Value of Cancer Drugs (CanREValue) collaboration. BMJ Open. 2020 Jan 7;10(1):e032884. DOI: 10.1136/bmjopen-2019-032884
- Clausen M. Use of real-world evidence in cancer drug funding decisions in Canada: a qualitative study of stakeholders’ perspectives. CMAJ Open. 2020 Nov 24;8(4):E772-E778. doi: 10.9778/cmajo.20200118.
- EU Framework for RWE Real World Evidence and Regulatory Decision Making Joint CSPS and Health Canada Workshop Presented by Dr Peter Arlett 3 December 2019 Head of Pharmacovigilance and Epidemiology, EMA (http://www.cspscanada.org/wp-content/uploads/ARLETT-Peter-Session-1-European-Medicines-Framework-for-RWE.pdf )
- LSE. RWE in Europe Paper V Policy challenges around Real World Evidence adoption in Europe. Dec 2018 (https://www.lse.ac.uk/business-and-consultancy/consulting/assets/documents/rwe-in-europe-paper-v.pdf )
- Bullement A, Podkonjak T, Robinson MJ, Benson E. Real-World Evidence Use in Assessments of Cancer Drugs by NICE. Int J Technol Assess Health Care. 2020; doi:10.1017/S0266462320000434
- Flatiron press release 14 July 2020. NICE Partners with Flatiron Health to Develop Real-World Evidence Research Methodologies. https://flatiron.com/press/press-release/nice-partnership-2020/ accessed 13 Feb 2021.
- Berger ML et al. Good practices for real-world data studies of treatment and/or comparative effectiveness: Recommendations from the joint ISPOR-ISPE Special Task Force on real-world evidence in health care decision making. Value Health. 2017 Sep;20(8):1003-1008. doi: 10.1016/j.jval.2017.08.3019.
- Orsini LS et al. Improving Transparency to Build Trust in Real-World Secondary Data Studies for Hypothesis Testing-Why, What, and How: Recommendations and a Road Map from the Real-World Evidence Transparency Initiative. Value Health. 2020 Sep;23(9):1128-1136. doi: 10.1016/j.jval.2020.04.002.
- Roberts MH and Ferguson GT. Real‑World Evidence: Bridging Gaps in Evidence to Guide Payer Decisions. PharmacoEconomics Open 2021;5:3-11 https://doi.org/10.1007/s41669-020-00221-y
- Oortwijn W et al. How to Deal with the Inevitable: Generating Real-World Data and Using Real-World Evidence for HTA Purposes – From Theory to Action. Int J Technol Assess Health Care. 2019;35(4):346-350. doi: 10.1017/S0266462319000400.
- “There is a difference” from Big data in healthcare (2018), available at: catalyst.nejm.org/doi/full/10.1056/CAT.18.0290
- Dash et al. Big data in healthcare: management, analysis and future prospects. Journal of Big Data 2019;6:54. journalofbigdata.springeropen.com/articles/10.1186/s40537-019-0217-0
Image by Erika Varga from Pixabay
