Advanced Therapy Medicinal Products (ATMPs) encompass gene, somatic cell therapies and tissue-engineered products, as well as these in combination with a medical device. Refer to classification decision tree of AMTPs for further detail.
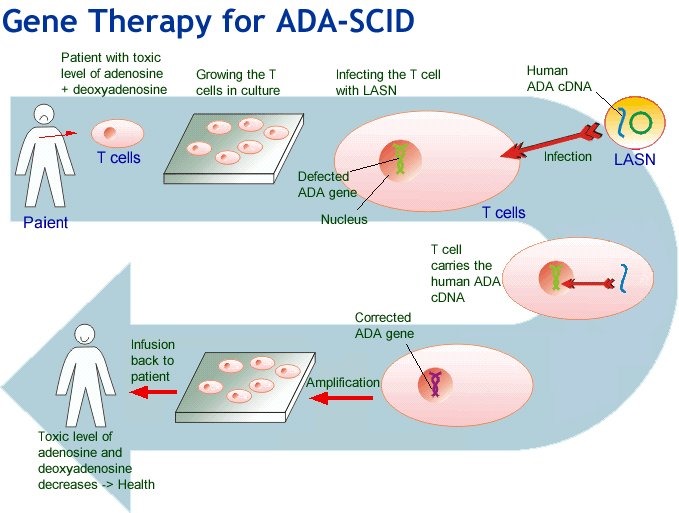
In general, these products involve replacement or regeneration of human cells, tissues or organs in the ultimate personalised medicine. Many also have the potential for a one-time cure. For example, GSK’s Strimvelis for treatment of adenosine deaminase deficiency–severe combined immunodeficiency (ADA–SCID), referred to as ‘bubble boy’ syndrome (below).
Regulation of ATMPs
The legal and regulatory framework for ATMPs in the European Union was formalised under Regulation (EC) No 1394/2007 over a decade ago. Since June 2009, the primary responsibility of the Committee for Advanced Therapies (CAT) is to assess the quality, safety and efficacy of ATMPs, and to follow scientific developments in the field on behalf of the European Medicines Agency. A summary of work to date is available in this presentation by the CAT Secretariat (May 2018). New guidelines on GMP and GCP for ATMPs have also been developed.
Meanwhile, the US FDA Center for Biologics Evaluation and Research (CBER) Office of Tissue and Advanced Therapies (OTAT, formerly known as the Office of Cellular, Tissue and Gene Therapies, or OCTGT) was renamed out of a restructure in 2016. In August 2017, the FDA Commissioner Scott Gottlieb, M.D. issued a statement on the agency’s new policy steps and enforcement efforts to ensure proper oversight of stem cell therapies and regenerative medicine. This has been followed by a further statement in July 2018 focused on gene therapies and ongoing releases of guidelines for consultation.
The International Pharmaceutical Regulators Forum’s (IPRF) currently has working groups for Gene Therapy and Cell Therapy to foster broad consultation and harmonisation. Identified challenges include the small quantities generated, often autologously, and short shelf-lives which do not fit with GMP principles. Similarly, without suitable animal models how is non-clinical testing to be performed, and does it need to be? Additionally, clinical trial design and data collection will require flexibility due to the rarity of most of the conditions being treated.
Reimbursement of ATMPs
In 2017, NICE commissioned a mock appraisal of ATMPs which concluded: ‘(1) the existing appraisal methods and decision framework were applicable to regenerative medicines; (2) quantification of decision uncertainty was key in decision-making; (3) where uncertainty is substantial, innovative payment mechanisms may play an important role and facilitate timely patient access; and (4) choice of discount rate is extremely important and can have an impact on the incremental cost-effectiveness ratio (ICER).‘ GSKs Strimvelis® received a positive NICE recommendation in October 2017 as an option for treating ADA–SCID when no suitable human leukocyte antigen-matched related stem cell donor is available. The cost at the time was Euro 594,000 (approximately AU$ 1 million) for the one-off treatment.
In a recent open access article, Jonsson and colleagues describe the convening of an Expert Panel to identify and discuss potential issues for ATMPs when evaluated using Health Technology Assessment (HTA) frameworks. Identified challenges included clinical evidence generation, safety concerns, assessing and paying for value, uncertainty, affordability, and the manufacturing and organisation of service delivery. They prioritised three topics similar to those considered important by the NICE appraisal described above:
- Uncertainty – due to the nature of evidence likely to be available for ATMPs. Potential solutions posed include measuring survival separately for cured and non-cured patients. They reference an article by Othus et al. 2017 which uses this approach for ipilimumab in melanoma and reduces the ICER by a third. Collection of real world evidence and outcome based agreements, or leasing schemes are also discussed.
- Discounting – cost-effectiveness estimates for one-off treatments are sensitive to distribution of costs and benefits. ATMPs are likely to involve high treatment costs occurring years before all health benefits accrue, and it is this time period that is sensitive to discount rates and thus estimates of cost-effectiveness. They consider if specific discounting rules should apply to ATMPs reflecting on the opportunity cost of capital, time preference of stakeholders, and decreasing marginal utility of income and conclude that a lower discount rate should be used to support technologies with costs now and outcomes in the long-term, as well as benefiting future generations.
- Health outcomes and value when considering potential curative treatment for a few versus smaller incremental benefits for a much larger population. They discuss ‘priority setting’, how current HTA frameworks do not fully capture stakeholder values, QALYs and thresholds for decision making and limitations on how to account for other factors. They point out that ‘guidelines for reimbursement are silent about the role of empirical studies for assessing the value of specific products or classes of products from patients or the general public.’ Those who work in the HTA space know that other factors may also be relevant for payers, patients, and society in addition to health gain and health system costs.
The article concludes with recommendations as a means to initiate and continue discussion. Interestingly, the first four, of eight ATMPs currently EC-approved (ChondroCelect®, Glybera®, MACI®, Provenge®) have all been withdrawn from market for commercial reasons. After 15 years of research and development involving 500 patients, ChondroCelect® received marketing approval in the EU in 2009. It was voluntarily withdrawn by the sponsor in 2016.

The challenges faced by ATMPs include complex and expensive regulatory and manufacturing processes, small patient populations with limited clinical data, and the need for high upfront investment for a course of therapy that may only be a single treatment. As with current therapies for rare and ultra-rare diseases, how society can provide equitable patient access at the same time as commercial returns will be an ongoing debate.
Sources: AT-MP; Flow diagram