Streamlining PBS processes – initial submission categories
While the “Streamlining PBS Processes” work has completed, and the Strategic Agreement 2017-2022 of which it was a part, has passed into history, what is the ongoing impact?
The new classification of submission types became effective from the PBAC July 2021 Meeting (March 2021 cut-off). This meant the loss of the ‘major’ and ‘minor’ labels traditionally used to distinguish between those submissions requiring full evaluation and those managed at Committee Secretarial level. Definitions applying to both new submissions and re-submissions have been changed.
For new submissions, five categories apply based upon the complexity of the submission and evaluation required. The new processes also apply to vaccine listings on the National Immunisation Program (NIP).
Categories 1 and 2, formerly major submissions, require the PBAC to assess the magnitude of clinical improvement or toxicity reduction, the incremental cost and the comparative costs and outcomes where an economic evaluation is required to support a claim of cost-effectiveness, cost-utility or cost‑minimisation.
Categories 3, 4 and Committee Secretariat, formerly minor submissions do not require a full evaluation (clinical, economic and financial evaluation).
The chart shown includes submissions by category for formal and out-of-session PBAC meetings held in the 12 months from July 2021. This encompasses the three formal meetings of July 2021, November 2021 and March 2022; and three out-of-session, September 2021, December 2021 and May 2022. Unfortunately, despite 14 considerations at the May 2022 meeting, these were not classified during the new terms. For the purpose of analyses, these are assumed to be resubmissions.
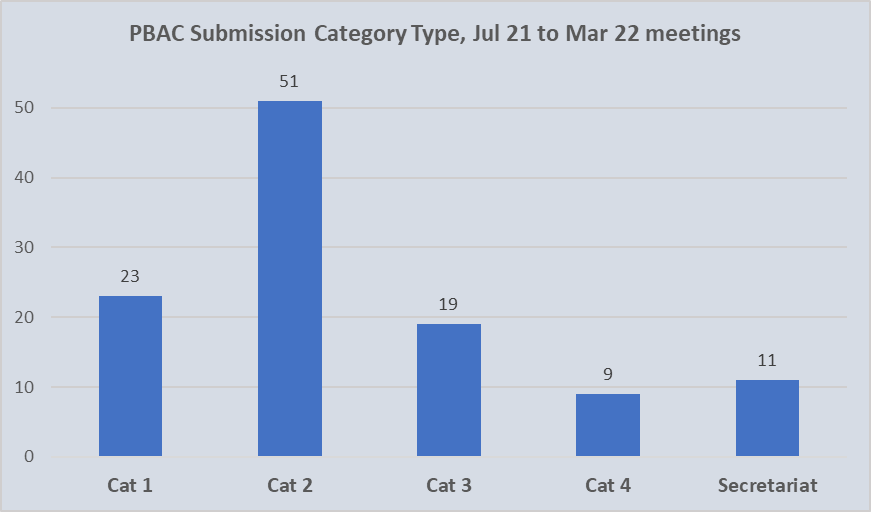
Of the 113 initial submissions categorised over the 12-month period, 74 (66%) were allocated as Category 1 or Category 2. Of these, approximately one third (23) qualified as Category 1 with an associated complexity or innovation. Given that overall, Category 1 submissions accounted for 21% of all those lodged, it does not reflect a decrease in novel pharmacological treatments being brought to Australia.
Cost-recovery
Interestingly, it is The National Health (Pharmaceuticals and Vaccines – Cost Recovery) Regulations 2022 that “prescribes the requirement for categorising submissions”. However, in relation to cost recovery matters, nothing takes precedence over the Cost Recovery Regulations.
Using the recently announced revised Cost Recovery Implementation Statement (CRIS), effective 1 August 2022, fees of approximately AU$ 15 million would have been received by the Commonwealth Department of Health and Aged Care over the 12 month period for new submissions. Fees for subsequent submissions and other services are not included in this total.
Category definitions
Category 1
- A first in class medicine and/or a medicine for a new population OR
- A drug with a codependent technology that requires an integrated codependent submission to the PBAC and MSAC OR
- A drug with a TGA Provisional determination related to the proposed population.
Category 2
Category 2 is the default for submissions requesting a PBS listing that does not meet the criteria for Category 1.
- A new medicine, a new indication of a currently listed medicine, or to make one or more changes to an existing listing that requires a full evaluation (clinical, economic and financial evaluation).
- A request for the PBAC to reconsider an existing recommendation where there is a change to the clinical, economic and/or financial information most recently relied on by the PBAC.
- A new form or strength of an already-listed medicine that is not bioequivalent to an existing listed form of the medicine. A Category 2 submission may be necessary to demonstrate that the new form delivers similar clinical outcomes to the existing form.
Category 3
Category 3 submissions relate to requests to change existing listings that do not change the population or cost-effectiveness of the medicine AND that do not meet the criteria for a Category 4 submission.
Although the PBAC will assess the clinical need for and clinical effectiveness of the requested listing, an economic evaluation is not necessary to support the claims made in the submission. Additionally, the financial estimates do not require the PBAC to assess any substantial financial implications for the supply of a listed medicine or designated vaccine.
They may also relate to a request for the PBAC to reconsider an existing recommendation where there is no change to the clinical, economic or financial information most recently relied on by the PBAC.
As PBAC advice is required on a case-by-case basis regarding the potential for schedule equivalence for biosimilar listings, Category 3 submissions are also appropriate for a new biosimilar brand of an existing pharmaceutical item with no indication changes.
Category 4
Category 4 submissions involve a request for one or more of the following:
- Listing of a new pharmaceutical item of a listed medicine.
- Consideration as an exempt item (Exempt item as per subsection 84AH of the National Health Act 1953).
- Varying or including a listed medicine on the prescriber bag.
- A change to the existing, or the addition of a new form or manner of administration of a listed medicine.
- A change to the maximum quantity and/or number of repeats of a listed medicine.
- A change or addition to the prescriber type(s) of a listed medicine.
Committee Secretariat
Committee secretariat submissions relate to applications where the requested listing changes do not require the PBAC to consider comparative effectiveness, cost-effectiveness or clinical need:
- there is no difference in patient safety or population for the new pharmaceutical item in the submission compared to an already-listed pharmaceutical item; AND
- there is no financial effect associated with the proposed change to the PBS.
Note: Applications that do not require PBAC consideration for listing an additional brand (a generic medicine) or new oral form of an existing TGA-approved and PBS-listed pharmaceutical item should be lodged directly to the Department of Health &Aged Care.
References
Procedure guidance for listing medicines on the Pharmaceutical Benefits Scheme version 2.4



