Are Rebates about to become extinct?
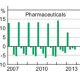
The Federal Government has proposed a major change to the existing process whereby manufacturers of PBS-listed ‘special pricing arrangements apply’ items are currently reimbursed. The graph, taken from this year’s Medicines Australia Federal Budget Submission, shows all the key elements in the debate.
The blue columns represent the reported PBS Commonwealth Government spend (left axis in AU $ millions) based on prices of medicines published in the PBS schedule, and paid through the supply chain. The red columns are the final net Government expenditure on the PBS, once manufacturer companies have repaid the difference between a medicine’s ‘published price’ (in the PBS Schedule) and the ‘effective price’ (the real price the Government is paying for a medicine, as negotiated with the manufacturer in a confidential Deed of Agreement). The green columns show the amount paid to manufacturers for a medicine after the margins and fees associated with movement through the supply chain, approximately 30%, are removed.
Hepatitis C drugs and their impact
Until the March 2016 PBS listing of new drugs for Hepatitis C, the annual difference between the two amounts (represented by the black line, right axis also millions with different scale) was relatively small. This was of minimal consequence to Government budgeting if the ‘additional’ spend occurred in one financial year but was not repaid until the next. Not so now that the total annual rebate amount has reached over AU $3 Billion.
Instead of having such a substantial sum owing, the Government wants to use this money to fund existing or new programs, especially with an election year approaching. An alternate way to view the delay in rebate repayment is as the cost to Australia of ensuring early access to life saving medicines. Is the health of Australians being risked for political reasons? In the case of the Hepatitis C drugs, at the effective price negotiated by the Australian Government they are so cost-effective, it is literally the deal of a lifetime, even with a lag in the receipt of rebates.
The reason for this complicated arrangement is to enable Australian patients to access new medicines in a timely manner. It comes into play when the price that the Australian Government requests for PBS listing is lower than the minimum purchase price required by a sponsor company. Keeping the real price paid confidential, or agreeing to repay some or all costs above a threshold, are ways to gain parent company permission for listing.
Do Medicines come to Australia early?
Registering a new medicine is a resource intensive process, and companies stagger the roll-out by market. Invariably, the FDA dossier is prepared and submitted first, followed by Europe. Australia is usually in the next group of countries to receive registration support. Parallel processing, whereby a reimbursement submission may be lodged and evaluated at the same time as the TGA evaluation occurs, means that Australia is frequently one of the first HTA countries to consider subsidy of a new medicine.
Globalisation impacts pharmaceutical pricing. Being early, the Australian published price is used by many other jurisdictions as a reference price. As such, PBAC considerations and subsequent PBS listings, are carefully observed, and have influence, globally.
[Part 2 will discuss Potential consequences]