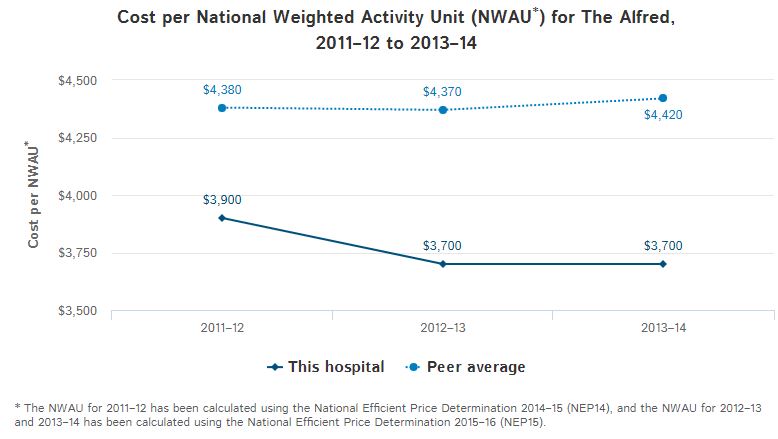
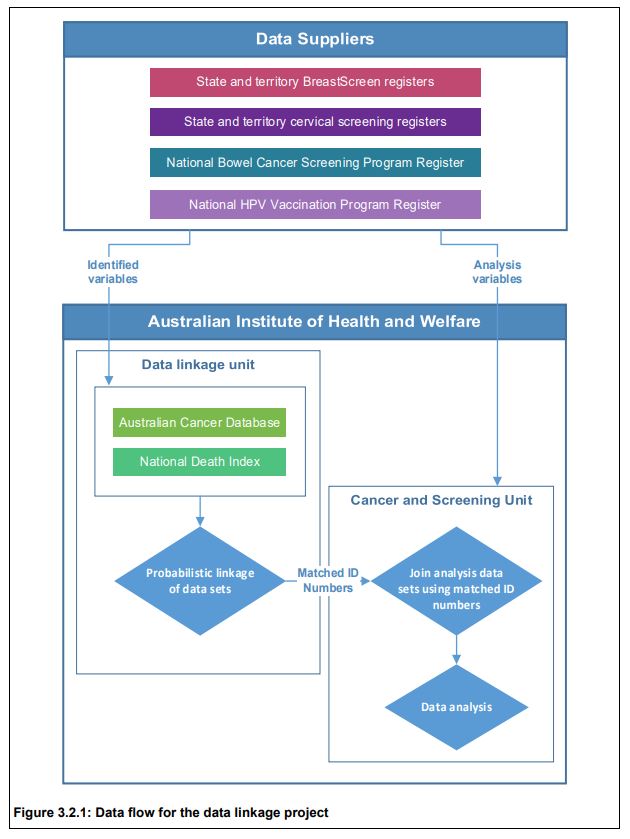
Evidence-Based Policy Making
Those working in the pharmaceutical industry are very familiar with evidence-based medicine (EBM) and evidence-based decision making for reimbursement. As such, we have come to expect that therapeutic and funding choices will be made following a systematic assessment of the available data. In the public policy arena however, that there can, and should be, a direct link between ‘the evidence’ and policy decisions is somewhat more problematic. Research (limiting to scientific research in this context) and policy worlds are very different.
Priorities and outcomes
The purpose of research is to advance knowledge, while policymakers want practical solutions. The research available may not necessarily be what policy makers want. From a design perspective, while control arms and blinding are de rigueur for scientific research, it may not be ethical or even possible to test political subjects using these methods. Until recently, access to operational data held by Government agencies was often difficult for researchers. Scientists rewards are focused on quality and resulting publication, less so on how findings may contribute to the public good.
Timing
Policy is delivered continuously. Demand for research is unpredictable and sporadic, with Government officials and politicians looking for it at particular moments – during campaigns, after scandals, failures, changes of leadership. Researchers must anticipate, and be ready with completed studies. However, research timelines are often long, with funding and approval lead times that make them quite unresponsive to spikes in demand. So the needed research is often not available at the time when it is required. Unfortunately, the constant shifting sands of politics may also make a well-constructed study obsolete before it even gets started.
The recent call by the Minister of Health for a reformatting of, and improved access to, Consumer Medicines Information (CMI) is one of those cases where the research has been done. Prompted by similar discussions in 2016, and ongoing efforts by industry to move away from the burdensome requirement for pack-based copies and the associated version issues, relevant data and consensus building has been undertaken. This is the ideal scenario, where the relevant policymaker or stakeholder can immediately send the Minister’s office a brief outlining a preferred solution.
A matter of perspective
Although you will see the term ‘evidence-based policy making’ (EBPM), the Blair UK and Rudd Governments were proponents, generally, it is considered aspirational, rather than a good description of the policy process. Those on the research side, may allege that policymakers ignore, do not understand nor act on the correct evidence. Why don’t policymakers select the most effective, evidence-based solution? This is because they are interpreting ‘evidence-based’ from the same frame in which they view scientific data.
Conversely, those familiar with policy making know what a messy process it can be, and have no expectation that evidence-based policy making will be solely reliant on data. It is an overarching term that refers to the consideration of a broad range of research evidence (from general public and other stakeholders; and evidence from practice and policy implementation) as part of a decision-making process that also incorporates other relevant factors such as political realities, and public sentiment.
As in science, the availability of policy research can also be fraught, especially if those in the debate are not skilled in the use of evidence, or it is presented selectively. For example, the advocacy for access to medicinal cannabis on the basis of a small number of patients successfully treated, when the scientific evidence is insufficient on which to base a treatment benefit:risk decision. In this case, other factors including Federal party politics and potential economic gains for the states contributed to policy decisions.
A matter of approach
While science is based on rational thinking, policy literature refers to a concept known as ‘bounded rationality’, jargon that describes what really happens when policy makers have ‘unclear aims, limited information, and unclear choices’. The term may come in handy next time you need to explain what turns out to have been a poor decision! Perhaps a new synonym for ‘uncertainty’? Policymakers use imperfect, and often ‘gut’ or emotion-based, short cuts to gather information and make decisions within a complex political environment.
Hence, it is unlikely that the robust scientific research which supports the development and introduction of a new medicine will be used in the same way when constructing public policy. While evidence plays a part in what is considered ‘good policy process’, it is in no way the whole show.
References available on request. Picture source